CONCLUSIONS
Imetelstat, an oligonucleotide and a first-in-class telomerase inhibitor, selectively targets malignant hematopoietic stem and progenitor cells with high telomerase activity by direct binding to the RNA template. In patients with MDS the on-target effects of imetelstat are associated with the development of hematologic treatment-emergent adverse events (TEAEs). In the IMerge phase 3 trial, among patients treated with imetelstat, 68% experienced grade 3-4 neutropenia, and 62% had grade 3-4 thrombocytopenia; however, clinical consequences of grade 3-4 infection or bleeding were similar in patients treated with imetelstat and placebo (Platzbecker et al. EHA 2023. Abstr S165). Here, we report additional data on the occurrence and management of cytopenias after treatment with imetelstat.
IMerge phase 3 trial (NCT02598661) is a multicenter study involving 178 patients with a median age of 72 years with red blood cell (RBC) transfusion dependency and LR-MDS relapsed/refractory to or ineligible for erythropoiesis-stimulating agents. Patients were randomized 2:1 to receive either imetelstat or placebo and stratified by prior RBC transfusion burden (4-6 or >6 U) and by International Prognostic Scoring System risk group. The safety population included all patients who received ≥1 dose of study drug and comprised 118 patients treated with imetelstat and 59 with placebo.
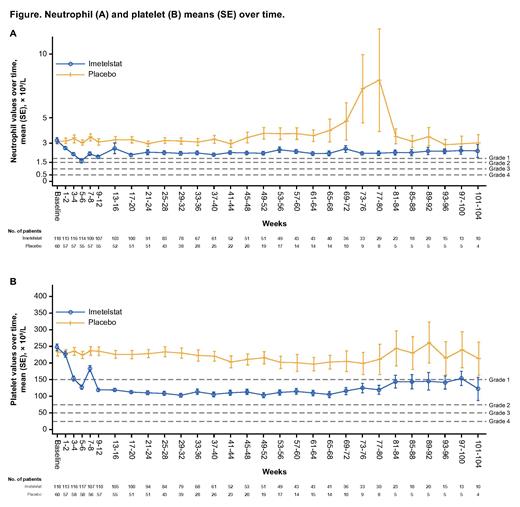
TEAEs of neutropenia and thrombocytopenia with imetelstat treatment were more prevalent in cycles 1-3 (68.6% and 62.7% respectively), and their frequency decreased over time: 42.7% and 48.5% in cycles 4-6, 36.8% and 44.7% in cycles 7-12, and 31.3% and 37.5% in cycle 13 and beyond. Based on laboratory assessment, neutrophil and platelet counts decreased from baseline levels in patients treated with imetelstat versus those treated with placebo (91.5% and 95.8% vs 47.5% and 33.9%, respectively). Neutropenia decreased to a maximum of grade 4 and 3 in 55 and 29 of imetelstat-treated patients, respectively, and >80% of cytopenia events stabilized to grade ≤2 within 4 weeks (Figure). The median time to grade 4 neutropenia was 5.0 weeks (range,1.1-90.3 weeks) for the imetelstat group vs 23.0 weeks (range, 23.0-23.0 weeks) for the placebo group. Similarly, median time to grade 4 thrombocytopenia was 5.2 weeks (range, 2.0-29.9 weeks) with imetelstat vs 35.5 weeks (range, 28.0-43.0 weeks) with placebo. It is noteworthy that among 47 patients who achieved the primary end point of 8-week RBC transfusion independence with imetelstat, 34 (72.3%) and 28 patients (59.6%) had grade 3-4 neutropenia and grade 3-4 thrombocytopenia, respectively.
In the imetelstat group, 3 patients had grade 3-4 neutropenia concurrent with grade 3-4 infections, and no patients experienced grade 3-4 thrombocytopenia associated with grade 3-4 bleeding events. There were no grade 5 cytopenias for either imetelstat or placebo groups.
In the imetelstat group, cytopenias were managed with protocol-specified treatment delays and dose adjustments. Dose reductions due to neutropenia and thrombocytopenia occurred in 39 patients (33.1%) and 27 patients (22.9%), respectively. Of imetelstat-treated patients, 6 (5.1%) discontinued treatment due to neutropenia (1 case, grade 4; all others, grade 3), and 4 (3.4%) discontinued due to thrombocytopenia (1 case, grade 2; 3 cases, grade 3). In addition to dose reductions and discontinuation of imetelstat, thrombocytopenia and neutropenia were managed by cycle delays in 46.6% and 50.8% of patients, by platelet transfusions in 17.8% of patients, and by concomitant therapy with growth factor support (mostly during cycles 2-4) in 34.7% of patients. Similar patterns of imetelstat dose modifications were seen in a pooled phase 2 and phase 3 safety analysis of IMerge.
In the IMerge phase 3 trial, thrombocytopenia and neutropenia were the most common and most frequently reported grade 3-4 AEs during treatment cycles 1-3. However, these AEs were generally transient, reversible, and manageable through treatment delays and dose adjustments, allowing for patients to remain on treatment and continue to experience clinical benefit. Moreover, occurrence of grade 3-4 cytopenias in responders suggests that these TEAEs do not affect the efficacy of imetelstat.
OffLabel Disclosure:
Zeidan:Tyme: Consultancy, Honoraria; Astex: Research Funding; Foran: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Shattuck Labs: Research Funding; Otsuka: Consultancy, Honoraria; Geron: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria. Savona:Taiho: Consultancy; Sierra Oncology: Consultancy; Ryvu: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy; Karyopharm: Consultancy, Current equity holder in publicly-traded company; Geron: Consultancy; Forma: Consultancy; BMS/Celgene: Consultancy; AbbVie: Consultancy; TG Therapeutics: Research Funding; Takeda: Research Funding; Incyte: Research Funding; Astex: Research Funding; ALX Oncology: Research Funding; Takeda: Consultancy; TG Therapeutics: Consultancy. Madanat:Sierra Oncology: Honoraria; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; Morphosys: Honoraria, Other: travel reimbursement; GERON: Consultancy; Taiho oncology: Honoraria; Novartis: Honoraria; OncLive: Honoraria; MD Education: Honoraria; Rigel Pharmaceuticals: Honoraria; Stemline therapeutics: Honoraria. Fenaux:Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; French MDS Group: Honoraria; Jazz: Consultancy, Honoraria, Research Funding. Komrokji:AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy. Jonášová:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: received travel, accommodations, and expenses ; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: received travel, accommodations, and expenses ; Novartis: Consultancy, Speakers Bureau. Illmer:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sun:Geron: Current Employment, Current equity holder in publicly-traded company. Berry:Geron: Current Employment, Current equity holder in publicly-traded company. Feller:Geron: Current Employment, Current equity holder in publicly-traded company. Navada:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Santini:Janssen: Other: travel grant; CTI: Other: Advisory boards; Geron: Other: Advisory boards; Gilead: Other: Advisory boards; BMS/Celgene: Other: Advisory boards; Novartis: Other: Advisory boards; Otsuka: Other: Advisory boards; Servier: Other: Advisory boards; Syros: Other: Advisory boards; AbbVie: Other: Advisory boards. Platzbecker:Janssen Biotech: Consultancy, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Curis: Consultancy, Research Funding; Fibrogen: Research Funding; AbbVie: Consultancy; Geron: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Celgene: Honoraria; Syros: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding.
Imetelstat to treat lower-risk myelodysplastic syndromes relapsed/refractory to or ineligible for erythropoiesis-stimulating agents

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal